By
Alex Müller
Published: Oct. 3, 2011, 1:34 p.m.·
Tags:
Diagnostics
The Gene Xpert is a new test for tuberculosis. It can find out if a person is infected with TB, and also if the TB bacterium of the person has resistance to one of the common TB drugs, rifampicin.
Read More →
By
Joan Leavens
Published: Sept. 13, 2011, 10:53 a.m.·
Tags:
Treatment

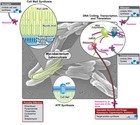
TMC207 (also known as bedaquiline, R207910 or the ‘J’ compound) is an experimental anti-TB drug. Discovered by Johnson & Johnson, TMC207 is the first compound in a new class of potent anti-TB drugs, the first new class in 60 years. Studies have shown that it is effective against both drug-resistant and susceptible TB. A recently completed phase II trial found that it reduces the time it takes for sputum to become negative in patients, meaning that it has the potential to shorten the duration of TB treatment. Clinical phase II trials are currently being carried out to evaluate the effectiveness of TMC207 for TB treatment.
How it works
------------
TMC207 is categorized as a diarylquinoline. This is an entirely new class of TB drug that works by inhibiting an enzyme that is vital for the production of energy, or ATP, in TB bacteria. This enzyme is called ATPase. TMC207 binds to ATPase and prevents it from supplying energy for the bacterial cell, which kills the bacterium.
Pre-clinical trials
-------------------
Researchers at Johnson & Johnson published their first report on TMC207 in 2005. They found that TMC207 was effective against both drug-sensitive (i.e. non-resistant) and drug-resistant TB bacteria in vitro. In mice, the drug was found to exceed the effectiveness of isoniazid and rifampicin when taken alone. When substituted for first-line drugs in the standard treatment programme, the activity of each new combination with TMC207 was improved.[^Andries]
A study published in 2006 examined TMC207 as a treatment for MDR TB using the mouse model. Mice were treated with various combinations of TMC207 with the standard regimen of second-line drugs (amikacin, pyrazinamide, moxifloxacin, and ethionamide). Combinations that included TMC207 were found to be more effective against MDR TB than the current regimen in nearly every case.[^Lounis]
A study in 2008 examined the activity of TMC207 against TB bacteria in mice lungs. Most notably, the study found that using a triple combination of TMC207 with rifapentine and pyrazinamide achieved outstanding bactericidal activity, with lung culture negativity in 9 of 10 mice.[^Veziris]
These early studies revealed some potentially important attributes of TMC207. Firstly, the mouse model suggested a synergistic interaction between TMC207 and pyrazinamide, meaning that these drugs may be more powerful when given in combination than either drug alone. More studies are needed to investigate this. Secondly, the drug showed activity against both drug-resistant and non drug-susceptible strains of TB, meaning that it could work as a treatment for MDR / XDR TB.
Additionally, in-vitro studies of TMC207 showed that the drug is a potent sterilizing agent, meaning that it is able to effectively eliminate TB bacteria. If the behavior of TB bacteria has this same property in-vivo (in human patients), then TMC207 could shorten the duration of TB treatment. This would be a much-needed change to what is currently a very lengthy and cumbersome treatment programme. The sterilizing ability of TMC207 also might make it a powerful drug in the struggle to eradicate TB.[^Matteelli]
Phase I trials
--------------
A phase I trial, the first stage of testing in human patients, was completed in South Africa. Results were published in 2008. The study examined the Early Bactericidal Activity (EBA), meaning its activity against TB early on, in 75 different TB-infected patients who had not had prior TB treatment. Of these patients, 31% were HIV positive. For seven days, these patients took either 600mg rifampicin, 300mg isoniazid, or a particular dose of TMC207. Researchers found that the bactericidal activity (i.e. ability to eliminate TB bacteria) of TMC207 at a dose of 7400 mg daily was similar to that of the other two first-line drugs, rifampicin and isoniazid. They found that TMC207 took slightly longer to start eliminating TB bacteria, with bactericidal effects beginning on day 4. In addition, the drug was well tolerated in patients, with no serious side effects.[^Rustomjee]
Another phase I trial is currently being carried out at University Hospitals (UH) Case Medical Center in the U.S. This trial will give TMC207 to 32 healthy individuals to test for the drug’s safety and tolerability. The study will also examine whether TMC207 has any drug interactions with other TB medications, such as rifabutin and rifampicin.[^TB Online]
Researchers believe that there may be a drug-drug interaction between TMC207 and rifampicin, which is of major concern given that rifampicin is a first-line TB drug. An enzyme (called CYP3A4) that metabolizes - and thereby activates - TMC207 is inhibited by rifampicin. This means that when both drugs are used together, rifampicin may prevent TMC207 from working properly. A study among 16 volunteers indicates that rifampicin might indeed make TMC207 less powerful.[^Lounis] More studies are needed to investigate this interaction.
Phase II trials
---------------
In a phase II trial, experimental drugs are given to a larger group of patients than in phase I. A phase II trial for TMC207 is currently being carried out in South Africa. The study is coordinated by teams of researchers at the Univ. of Stellenbosch, Univ. of Witwatersrand, Aurum Health, Medical Research Council, and Tibotec. This trial has two stages. The first stage has already been completed, and results were published in 2009. The purpose was to determine whether TMC207 was effective in reducing the time it took for patients to convert to sputum-negative. A group of 47 patients, all of whom were HIV negative and had been newly diagnosed with MDR TB, were randomly assigned to two groups. The first group received TMC207 at a dose of 400 mg daily for 2 weeks, followed by 200 mg three times a week for 6 weeks. The second group received the standard five-drug, second-line regimen for treating MDR TB, and a placebo was used instead of TMC207.
Researchers found that TMC207 reduced the time it took for sputum to convert to negative in patients. At the end of the trial, 9% of patients who took the placebo were sputum-negative, as compared to 48% of those who received TMC27. TMC207 eliminated TB bacteria more quickly, and it was shown to be safe and well tolerated in patients. The only side effect that was significantly more common in the group that took TMC207 was nausea (26% vs. 4%). Researchers concluded that “the clinical activity of TMC207 validates ATPsynthase as a viable target for the treatment of tuberculosis.”[^Diacon] The second stage of this two-part trial will be a multinational study of patients in South Africa, Peru, Latvia, India, Brazil, Thailand, The Philippines and Russia. Because the first part of the study showed that TMC207 is highly effective against TB, the study’s second stage will be open label and non-randomised.
Future developments
-------------------
Johnson & Johnson’s research subsidiary, Tibotec, is managing the clinical development of TMC207 to determine whether the drug can be used in the treatment of MDR / XDR TB. Tibotec will elaborate a program whereby developing countries can gain access to TMC207. In addition, Tibotec has given the TB Alliance a royalty-free license to develop TMC207 for drug-sensitive TB.
Current and future clinical trials of TMC207 will examine the potential use of TMC207 in the treatment of children with MDR TB; for the treatment of latent TB infection; for use in combination with antiretrovirals; and as a shortened treatment regimen for drug-sensitive TB.[^Matteelli]
Advocacy Issues
---------------
- More clinical information is needed on the use of TMC207 in TB patients with HIV co-infection.
- Organizations in South Africa have called for TMC207 to be made immediately available for
compassionate use. They recommend that clinicians in South Africa apply to the Medicines
Control Council (MCC) for Section 21 authorizations to use bedaquiline. These authorizations
are already being used to procure access to PAS, a less effective and harder to tolerate
medication than TMC207.[^TAC]
[^Andries]: K Andries et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis. Science. 2005 Jan 14; 307(5707): 223-7
[^Lounis]: N Lounis et al. Combinations of R207910 with Drugs Used To Treat Multidrug-Resistant Tuberculosis Have the Potential to Shorten Treatment Duration. Antimicrobial Agents and Chemotherapy. 2006 Nov; 50(11): 3543-3547.
[^Veziris]: N Veziris et al. A Once-Weekly R207910-Containing Regimen Exceeds Activity of the Standard Daily Regimen in Murine Tuberculosis. Am J Respir Crit Care Med. 2009 Jan 1; 179(1): 75-9
[^Matteelli]: A Matteelli et al. TMC207: the First Compound of a New Class of Potent Anti-Tuberculosis Drugs. Future Microbiol. 2010 June; 5(6): 849-858.
[^Rustomjee]: R Rustomjee et al. Early Bactericidal Activity and Pharmokinetics Of the Diarylquinoline TMC207 in Treatment of Pulmonary Tuberculosis. Antimicrob Agents Chemother. 2008 Aug; 52(8): 2831-5.
[^TB Online]: The Medical News. [TMC207 represents first new class of anti-TB drugs in the past 60 years](http://www.tbonline.info/posts/2011/8/1/uh-case-medical-center-commence-phase-1-clinical-t/ "UH Med Centre").
[^Lounis]: N Lounis. Impact of the interaction of R207910 with rifampin on the treatment of tuberculosis studied in the mouse model. Antimicrob Agents Chemother. 2008 Oct; 52(10):3568-72
[^Diacon]: AH Diacon et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009 Jun 4;360(23):2397-405
[^Matteelli]: A Matteelli et al. TMC207: the First Compound of a New Class of Potent Anti-Tuberculosis Drugs. Future Microbiol. 2010 June; 5(6): 849-858
[^TAC]: [Mobilize Against TB](http://www.tbonline.info/posts/2011/8/30/addressing-tb-crisis-south-africa/ "TAC").
Read More →
By
Joan Leavens
Published: Sept. 2, 2011, 12:01 p.m.·
Tags:
Treatment
The standard TB regimen is a six month course of antibiotics but the duration and drugs used may vary according to a patient’s age, type of TB infection, and whether they have been treated before. Treating TB takes longer than treating other types of bacterial infections because the bacteria that cause TB grow slowly, and die slowly. The standard six month course of treatment consists of two phases. The first phase lasts two months and is called the intensive phase. The second phase lasts four months and is called the continuous phase.
Read More →
By
Treatment Action Campaign
Published: Aug. 30, 2011, 5:08 p.m.·
Tags:
None
Statement by the Treatment Action Campaign, the HIV Clinicians Society of Southern Africa, Médecins Sans Frontières, SECTION27 (incorporating the AIDS Law Project), HIV i-Base, Wits Reproductive Health and HIV Institute (WRHI), Jhpiego and the Centre for the AIDS Programme of Research in South Africa (CAPRISA)
Read More →
By
Lihle Dlamini
Published: Aug. 17, 2011, 4:32 p.m.·
Tags:
Diagnostics
Lihle Dlamini is the Deputy General Secretary of the Treatment Action Campaign. Her HIV activism started in 2002 when she was diagnosed with TB and experienced the horrors of the South African public health system.
Read More →
By
Alex Müller
Published: Aug. 9, 2011, 3:29 p.m.·
Tags:
Diagnostics
This article descibes the tests that are used to diagnose active TB.
Read More →
Published: July 1, 2011, 12:53 p.m.·
Tags:
Treatment
Use this slideshow to see the different TB drugs and how they work.
Read More →
By
Nathan Geffen
Published: June 29, 2011, 2:01 p.m.·
Tags:
None
How old is TB? Where does it come from? What was our understanding of TB before the rise of scientific medicine? Read this history of TB to find out.
Read More →
By
Kay Kim
Published: May 31, 2011, 10:05 p.m.·
Tags:
Drug-resistant TB
Stigma, isolation and a high risk of death: these are the challenges facing most drug-resistant TB patients. This article explains drug-resistance and what is fueling it.
Read More →