The evolving role of advocacy in tuberculosis
Tuberculosis advocacy has changed the disease from a silent epidemic to one with many voices that seek increased political will and investment in research.
Tuberculosis advocacy has changed the disease from a silent epidemic to one with many voices that seek increased political will and investment in research (1). Tuberculosis advocacy has gained strength as a mechanism to improve research, increase access to new methods to fight tuberculosis, and involve communities in policy and programmatic decisions (2, 3).
In the past few decades advocacy efforts for tuberculosis have contrasted starkly with those for HIV/AIDS. By making their protests visible and engaging intellectually with scientists and policy makers, HIV activists changed the course of AIDS research and global access to treatment (4). However, few survivors of tuberculosis or other people affected by the disease have served as public voices for it, partly because of its curable nature, the vertical top-down orientation of efforts for tuberculosis control, the persistent stigma about tuberculosis worldwide, and the lack of funding to support community involvement in tuberculosis research and programmes. As a result, although the burden of tuberculosis remained large, and even grew in the wake of the HIV epidemic, communities were not empowered to engage in prevention and care for tuberculosis, much less to participate in research and development for new methods to fight the disease (3).
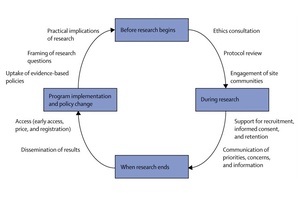
The advocacy movement for tuberculosis has grown as individuals and groups have developed their scientific literacy, becoming highly trained activists able to powerfully present community perspectives to scientists, policy makers, and industry. Public and non-profit sponsors of trials and other donors have begun to invest in advocacy. Guiding frameworks (eg, the Good Participatory Practice Guidelines for TB Drug Trials, 5) are now available to enable effective engagement with communities and stakeholders at all stages of the research process. By encouraging early and frequent engagement, the guidelines can ensure communities' needs are addressed in the research process. Affected communities now have many examples of how tuberculosis advocacy can affect decisions from trial design to programme implementation (figure) (1).
Involvement of tuberculosis activists in the research process at the outset of trial design is one of the most direct methods to ensure that research addresses the priorities of affected communities. This involvement can include framing of the research question and revision of early drafts of protocols after a research question is developed. For example, the Global TB Community Advisory Board, an independent group of highly research-literate activists from global networks for HIV and tuberculosis, identified as an important concern for patients that bedaquiline (Janssen Therapeutics, Titusville, NJ, USA) and delamanid, the two newest drugs to fight drug-resistant tuberculosis, both cause QT prolongation. The advisory board called on the drugs' sponsors to cooperate to establish if the drugs are safe to use together; the US National Institutes of Health is now arranging a drug—drug interaction study.
After the design phase, advocates are uniquely positioned to affect the implementation of research, especially in ensuring effective communication between research sponsors and the communities hosting clinical trials. The Community Research Advisors Group, an independent advisory body, assists communication between investigators from the Tuberculosis Trials Consortium (TBTC) and local communities at all stages of research. Several members of the Community Research Advisors Group organise local community advisory boards to raise awareness of tuberculosis research in communities near TBTC sites. By empowering survivors of tuberculosis, affected family members, policy makers, religious leaders, and other community members to engage with study teams, these community advisory boards manifest the social value of tuberculosis research for communities. In addition to aiding recruitment, enrolment, and retention of participants in TBTC studies, community advisory boards could help communities to accept tuberculosis research as a long-lasting partnership extending beyond the duration of any individual trial.
When studies conclude and investigators publish results, tuberculosis activists can play a crucial part to ensure that participants receive and understand findings, and that research developments translate into practical changes for affected populations. For example, findings from publicly funded research showed that rifapentine could reduce treatment lengths and provide intermittent dosing for patients with tuberculosis (6, 7). After these trials, the advocacy organisation Treatment Action Group and partners from the public sector, academia, and other advocacy groups identified price as an obstacle to implementation of rifapentine-based treatment in the USA (8). While engaging with the drug's sponsor, the Treatment Action Group and partners administered a survey among US tuberculosis controllers in which price was the most cited barrier to use of rifapentine. The Treatment Action Group discussed this evidence with the drug company Sanofi US, which eventually voluntarily reduced the drug's price by 57%.
Tuberculosis advocates also play a prominent role in shaping of policy and control programmes. Health-care workers—a previously underused resource, who face a three times higher risk of contracting tuberculosis and up to five times higher risk of contracting drug-resistant tuberculosis than do the general population (9, 10)—are also highly empowered, because of their access to information and training and their ability to affect policy and implementation of health care. A growing community of health-care workers and students who are tuberculosis survivors, collectively known as TB Proof, are using dual perspectives and professional networks as platforms to call for improved tuberculosis prevention and accelerated access to urgently needed new regimens and vaccines.
Tuberculosis advocacy has recently increased at a rapid pace and achieved successful outcomes; this trend must continue to reach global targets for tuberculosis elimination (11). At present, only a few trial sponsors engage communities in research; concrete and systemic engagement with communities is therefore needed from all tuberculosis scientists at each step of the research and development process, particularly in the pretrial and post-trial phases. Mechanisms include dedicated funding for community engagement, establishment of networks for activists to communicate with each other at national and international levels, and a commitment by all stakeholders to make tuberculosis advocacy an equal partner of processes for research, development, and policy planning. An environment in which tuberculosis survivors and advocates can affect research and ensure access to lifesaving technologies needs respect for the independence, autonomy, and professionalism of activists, and the end of persistent stigma about the disease.
We thank Alicia Chou for providing information about the Good Participatory Practice Guidelines for TB Drug Trials and also for her feedback, and Marco Schito for his feedback. LRM is cochair of the Community Research Advisors Group (CRAG). The CRAG receives limited funds from the Centers for Disease Control and Prevention to support its work with the Tuberculosis Trials Consortium. LRM receives no compensation for her involvement with CRAG, and all CRAG activities are done on a volunteer basis. MF is employed by the Treatment Action Group and provides technical and administrative support to the CRAG; the Treatment Action Group has several funding sources. All other authors declare that they have no competing interests. No funding from the Centers for Disease Control and Prevention or other public funding was used for the creation of this Comment. The opinions expressed herein are those of the authors.
References
1 . Engaging communities in tuberculosis research. Lancet Infect Dis 2013; 13: 540-545. Summary | Full Text | PDF(83KB) | CrossRef | PubMed
2 . An urgent call for a stronger, louder voice for TB vaccine advocacy. Tuberculosis 2013; 93: 2777-2778. PubMed
3 . Patient empowerment in tuberculosis control: reflecting on past documented experiences. Trop Med Int Health 2007; 12: 873-885. CrossRef | PubMed
4 . From HIV to tuberculosis and back again: a tale of activism in 2 pandemics. Clin Infect Dis 2010; 50: S260-S266. CrossRef | PubMed
5 . Good participatory practice guidelines for TB drug trials. http://cptrinitiative.org/resources/gpp-tb-resource-document/. (accessed Jan 6, 2014)
6 Jindani A, Hatherill M, Charalambous S, et al. A multicentre randomized clinical trial to evaluate high-dose rifapentine with a quinolone for treatment of pulmonary TB: the RIFAQUIN trial. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA; Mar 3—6, 2013. 147LB.
7 Dorman S, Goldberg S, Feng P, et al. A dose-ranging study of daily rifapentine-containing regimens for intensive phase treatment of pulmonary TB: Tuberculosis Trials Consortium Study 29x. 44th Union World Conference on Lung Health; Paris, France; Nov 2, 2013. OP-230-02.
8 Deluca A, Lessem E, Wegener D, et al. Understanding barriers to rifapentine use in US tuberculosis programs to inform pricing advocacy. 18th Annual Conference of the Union—North America Region; Boston, MA, USA; Feb 28, 2014. F3.
9 . Tuberculosis among health care workers. Emerg Infect Dis 2011; 17: 488-494. PubMed
10 . High incidence of hospital admissions with multidrug resistant and extensively drug resistant tuberculosis among South African health care workers. Ann Intern Med 2010; 153: 516-522. CrossRef | PubMed
11 . Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: World Health Organization, 2014.
a Center for Tuberculosis Research, Johns Hopkins University, Baltimore, MD 21231, USA
b Treatment Action Group, New York, NY, USA
c National Tuberculosis Controllers Association, Smyrna, GA, USA
d Planeta Salud, Barcelona, Spain
e TB Proof, Somerset West, South Africa
Source: The Lancet Respiratory Medicine